When your body turns on its own pancreas, life changes overnight. Type 1 diabetes isn’t just about high blood sugar-it’s the result of your immune system attacking and destroying the insulin-producing cells in your pancreas. This isn’t a lifestyle issue. It’s not caused by eating too much sugar. It’s an autoimmune disease, and it happens to people of all ages, even adults who were never told they were at risk.
What Really Happens in the Pancreas?
Your pancreas has two jobs: making digestive enzymes and making insulin. In type 1 diabetes, only the insulin-making cells-called beta cells-are targeted. Your immune system mistakes them for invaders and sends in T-cells to destroy them. This process, called insulitis, can start years before you ever feel sick. By the time you’re thirsty, peeing constantly, and losing weight, you’ve already lost 80-90% of your beta cells.
Doctors can measure this damage with a simple blood test for C-peptide. If your level is below 0.2 nmol/L, your body is no longer making meaningful insulin. That’s why you need injections-your body can’t produce enough on its own. Unlike type 2 diabetes, where insulin resistance is the main problem, type 1 means you have almost zero insulin left. No exceptions. No shortcuts.
It’s Not Just One Disease
Not everyone with type 1 diabetes is the same. Some kids are diagnosed after a sudden illness, with blood sugar over 500 mg/dL and ketoacidosis. Others, especially adults, slowly lose beta cells over years. That’s called LADA-Latent Autoimmune Diabetes in Adults. Many of these people are misdiagnosed as type 2 because they’re over 30, not overweight, and don’t fit the stereotype. But their blood tests show autoantibodies-proof it’s autoimmune.
Studies show 12% of adults diagnosed with type 2 actually have type 1. And if you’re given metformin instead of insulin, your blood sugar won’t improve. That’s why testing for GAD65, IA-2, and ZnT8 autoantibodies matters. If you’re newly diagnosed and not responding to oral meds, ask for these tests. It changes everything.
Stages of Type 1 Diabetes-Before Symptoms Show
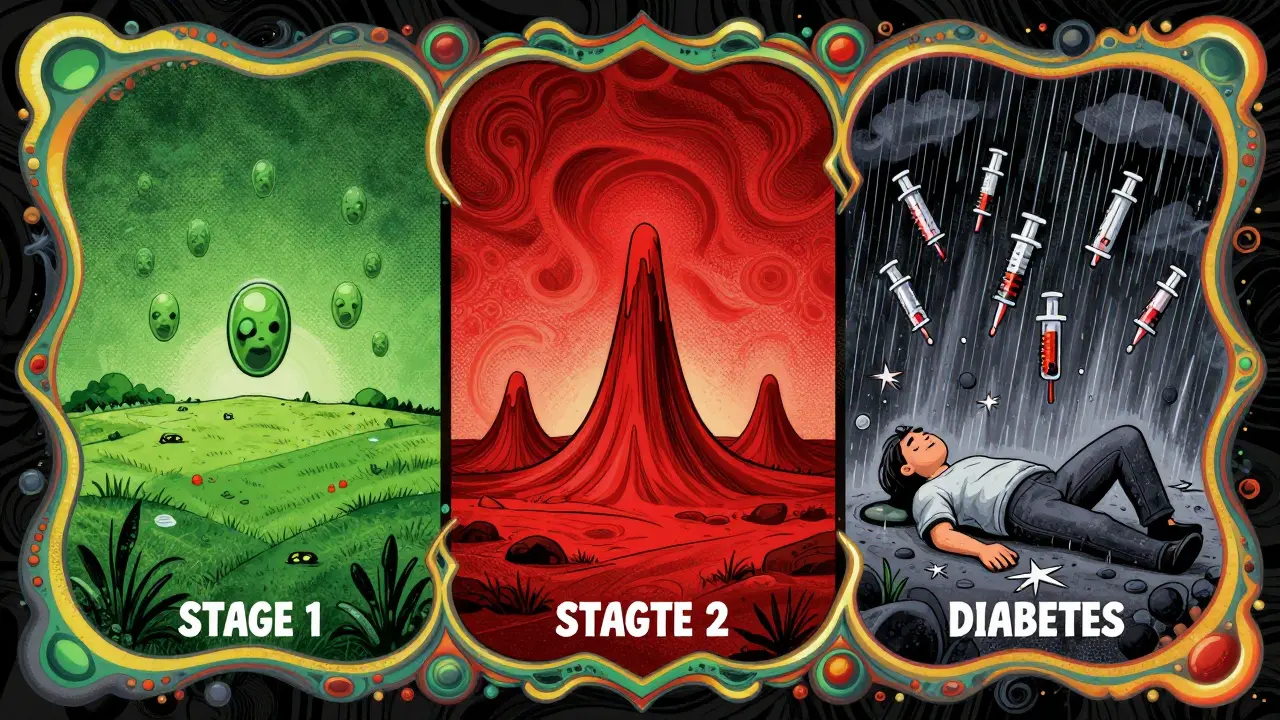
You don’t wake up one day with type 1 diabetes. It develops in three stages:
- Stage 1: You have two or more autoantibodies, but your blood sugar is normal. This happens in about 0.4% of the population-often without knowing.
- Stage 2: Your blood sugar starts to rise, but you still feel fine. This is the window where new treatments can help.
- Stage 3: Symptoms appear. You’re diagnosed. You need insulin.
The FDA-approved drug teplizumab (Tzield) can delay Stage 3 by over two years in people with Stage 2. It’s not a cure, but it buys time. And it’s only available if you’re tested early. If you have a family member with type 1, get screened. The TrialNet program offers free testing for relatives.
Genes, Viruses, and the Environment
Why does this happen? Genetics play a big role. If you carry the HLA-DR3/DR4 genes, your risk jumps 20 to 30 times higher. But not everyone with these genes gets diabetes. Something else triggers it.
Research points to viruses-especially coxsackievirus B. A 2019 study found people who later developed type 1 had higher levels of this virus in their blood months before diagnosis. It’s not that the virus causes diabetes. It’s that it may start the immune system down the wrong path in people who are already genetically prone.
Other factors like gut bacteria, vitamin D levels, and early diet (like when you introduced cow’s milk or gluten) are being studied. One 2022 study found people with type 1 often have less of a gut bacteria called Faecalibacterium prausnitzii, which helps reduce inflammation. Fixing your gut might not stop the disease, but it could slow it down.
Managing Blood Sugar-The Real Daily Work
Once diagnosed, your job is to replace what your pancreas lost. That means insulin. But not just any insulin. You need rapid-acting insulin before meals (like aspart or lispro) and long-acting insulin (like glargine U-300) to cover the baseline.
Most people start with 0.5 units of insulin per kilogram of body weight per day. Half is basal, half is bolus. But that’s just a starting point. You’ll adjust based on your meals, activity, stress, and sleep. No two people need the same doses.
Continuous glucose monitors (CGMs) like the Dexcom G7 have changed everything. They track your sugar every five minutes and send alerts when you’re dropping or spiking. In the 2023 DIAMOND trial, people using CGMs lowered their A1c by 0.4-0.6% and had 40-50% fewer low-blood-sugar episodes.
And then there’s the artificial pancreas-closed-loop systems like Tandem’s Control-IQ. These devices automatically adjust insulin based on your CGM readings. In one 2022 study, users spent 74% of the day in the target range (70-180 mg/dL). Without it? Just 51%. That’s a massive difference in energy, brain fog, and long-term risk.

The Rare Link: Autoimmune Pancreatitis
Here’s something few doctors talk about: sometimes, the immune system attacks not just the insulin cells, but the whole pancreas. Autoimmune pancreatitis (AIP) is rare-only about 1 in 300 people with type 1 diabetes also have it. But when it happens, it’s serious.
AIP affects the exocrine part of the pancreas-the part that makes digestive enzymes. People with both conditions may have belly pain, weight loss, or trouble digesting food. Their blood tests show high IgG4 levels. Treatment? Steroids. But steroids raise blood sugar. So if you’re on them, your insulin doses will need to go up.
If you have type 1 and suddenly start having digestive issues, ask your doctor about AIP. It’s not common, but missing it means you’re treating only half the problem.
What’s New? The Future of Treatment
There’s hope beyond insulin. In 2023, Vertex Pharmaceuticals reported that 89% of patients in a trial got insulin independence after receiving stem cell-derived islet cells. It’s still experimental, but it’s real. These cells are implanted in the liver and start making insulin like a real pancreas.
Another drug, verapamil-a cheap blood pressure pill-showed in a 2022 study that it preserved 30% more insulin production after one year. It’s not approved yet, but it’s being tested in larger trials.
And immunotherapies like abatacept are helping slow beta-cell loss in people recently diagnosed. In one trial, it cut C-peptide decline by 59% over two years. These aren’t cures, but they’re steps toward preserving what’s left.
The 2024 ADA/EASD guidelines now say the future of type 1 diabetes care is combination therapy: immunotherapy to stop the attack, plus drugs to protect or replace beta cells. That’s the new goal.
Costs, Challenges, and Real-Life Struggles
Living with type 1 diabetes is expensive. In the U.S., the average yearly cost is $19,743. Insulin alone costs $9,601 per person. Even with insurance, many people ration insulin because they can’t afford it. That’s deadly. No one should have to choose between food and their life-saving medication.
And the emotional toll? Constant monitoring. Fear of lows. The guilt when you have a high reading. The isolation. It’s not just a medical condition-it’s a 24/7 job. Support groups, therapy, and diabetes educators aren’t luxuries. They’re essential.
If you’re newly diagnosed, know this: you’re not broken. You’re not failing. You’re learning a new way to live. And you’re not alone. Over 1.25 million Americans are doing the same thing every day.
Can type 1 diabetes be reversed?
No, type 1 diabetes cannot be reversed. The immune system permanently destroys insulin-producing beta cells. However, new treatments like teplizumab can delay diagnosis in high-risk individuals, and stem cell therapies are showing promise in restoring insulin production. But these are not cures-they’re ways to slow progression or replace lost function.
Is type 1 diabetes the same as autoimmune pancreatitis?
No. Type 1 diabetes targets only the insulin-producing beta cells in the pancreas. Autoimmune pancreatitis attacks the exocrine part of the pancreas, which makes digestive enzymes. They’re separate diseases, but in rare cases (about 0.3%), both occur together. This is called a broader autoimmune pancreatic syndrome and requires treatment for both conditions.
Why do some adults get diagnosed with type 1 diabetes later in life?
This is called LADA-Latent Autoimmune Diabetes in Adults. The autoimmune attack on beta cells happens more slowly than in children. People with LADA may still produce some insulin for years after diagnosis, which is why they’re often misdiagnosed as type 2. But blood tests for autoantibodies confirm it’s type 1. They’ll eventually need insulin, but not always right away.
Do I need to take insulin forever?
Yes, if you have type 1 diabetes, you will need insulin for life. Your body no longer produces it. Even if you feel fine, skipping insulin leads to dangerous highs and life-threatening ketoacidosis. New therapies may reduce how much you need, but they don’t eliminate the need. Research into beta-cell replacement is promising, but it’s not yet widely available.
What’s the difference between type 1 and type 2 diabetes?
Type 1 is autoimmune-you lose your insulin-making cells. Type 2 is metabolic-you become resistant to insulin and your body doesn’t make enough over time. Type 1 requires insulin from day one. Type 2 can often be managed with diet, exercise, and pills-though many eventually need insulin too. The key test is C-peptide: low in type 1, normal or high in type 2.
Can I prevent type 1 diabetes if I have a family history?
You can’t prevent it entirely, but you can detect it early. If you have a close relative with type 1, you can get screened for autoantibodies through TrialNet. If you’re in Stage 1 or 2, you may qualify for teplizumab, which can delay diagnosis by over two years. Early detection gives you time to prepare and access new treatments before symptoms start.


So let me get this straight: I’m supposed to believe that someone who eats a donut every morning doesn’t get T1D, but someone who never touched sugar gets it because… their immune system had a bad day? 🤡 I mean, I get it’s autoimmune-but why does it feel like the medical world is trying to absolve everyone of responsibility while making patients feel like broken robots?
THE IMMUNE SYSTEM IS A TRAITOR. A. BLOODY. TRAITOR. 🚨 It doesn’t just turn on you-it GLOATINGLY DESTROYS your beta cells like they’re the last cookie in the jar and you didn’t even get to taste it. And then? They hand you a pen that costs more than your rent and say, ‘Here, live.’ I’m not just diabetic. I’m a walking clinical trial with a Dexcom on my arm and a grief counselor in my pocket.
While I appreciate the comprehensive overview, I must emphasize that the assertion regarding teplizumab’s efficacy is statistically sound but clinically nuanced. The delay in progression, while statistically significant (p<0.001), does not equate to disease modification in the immunological sense. Furthermore, the cost-benefit analysis remains contentious in resource-constrained settings. That said, the emergence of stem-cell-derived islets represents a paradigm shift-though scalability remains a formidable hurdle.
I’m from India and my cousin got diagnosed at 35. Everyone thought she had type 2. She was on metformin for 2 years before someone finally tested her for antibodies. When she got her diagnosis, she cried for 3 hours. But now? She’s on a CGM and actually sleeping through the night. Just… please, if you’re an adult and insulin isn’t working, ask for the test. It’s not that hard.
Let’s be real: the system is rigged. You get diagnosed, they hand you a pamphlet and a $500 insulin pen and say ‘good luck.’ Meanwhile, the same pharma companies that profit off your daily survival are lobbying against price caps. I’ve seen people ration insulin. I’ve seen people die. This isn’t medicine. It’s a hostage situation with a glucose meter.
I was diagnosed at 22. Thought I was gonna die. Now I’m 31, run marathons, and my CGM’s my best friend. The tech has saved my life-literally. I used to panic over every high. Now I just laugh and adjust. It’s not easy, but it’s doable. And if you’re new? You’re not alone. We’ve got your back. 💪
The way this disease sneaks up-like a ghost in your pancreas, whispering ‘I’m coming for you’ for years before the crash-it’s haunting. But here’s the wild part: the same immune system that attacks your beta cells? It’s also the one that fights off cancer. Maybe we’re not broken. Maybe we’re just… hypersensitive. Like our bodies are too alive for this world. And maybe that’s not a flaw. Maybe it’s a feature we just haven’t learned to channel yet.
Everyone’s talking about teplizumab and stem cells like it’s the holy grail but nobody’s talking about how 90% of T1D cases have zero family history. So if it’s genetic, why are most of us the first? And why do we all have different triggers? Viruses? Gut bugs? Maybe it’s not one disease. Maybe it’s 100 diseases pretending to be one.
I’ve lived with this for 27 years. I don’t need a cure. I need the system to stop treating me like a burden. My insulin is cheaper than my Netflix. My CGM costs more than my car. And I’m not asking for a miracle. Just a chance to live without choosing between food and my life.
It is of paramount importance to underscore that the pathophysiological cascade of autoimmune-mediated beta-cell destruction is not merely a localized phenomenon, but rather a systemic immune dysregulation involving intricate interplay between genetic susceptibility, environmental triggers, and microbiome modulation. The emergence of stage-based classification represents a monumental advancement in preventative medicine, yet its implementation remains hampered by inadequate screening infrastructure, particularly in low-resource regions where diagnostic latency remains tragically prevalent.
You say you’re not broken? You’re wrong. You’re a biological malfunction. A glitch in the human code. You don’t get to call yourself ‘not failing’ when your body is literally self-destructing. You’re living proof that evolution got lazy. And now you expect sympathy? Get over it. The world doesn’t owe you insulin.
In my village in Nigeria, we say: 'The body remembers what the mind forgets.' Type 1 is not just a disease-it is a whisper from your ancestors, a signal that your lineage carries a fire that does not burn out. The immune system does not attack without reason. Perhaps it is not a mistake, but a call-to awaken, to heal, to transform. We must not only treat the pancreas, but the soul that carries it.
The claim that 12% of adults diagnosed with type 2 actually have type 1 is statistically dubious. The autoantibody tests cited have false-positive rates up to 8% in non-clinical populations. Furthermore, the use of C-peptide as a definitive marker is misleading in the context of insulin resistance with partial beta-cell function. This post reads like a pharmaceutical marketing brochure disguised as medical education.