When you’re facing a cancer diagnosis that requires surgery, one of the most important decisions isn’t about the operation itself-it’s about when to give the drugs. Should treatment come before surgery to shrink the tumor? Or after, to clean up what’s left? This isn’t just a technical question. It’s personal. It affects how long you wait for surgery, how you feel during treatment, and even your chances of staying cancer-free years from now.
What’s the Difference Between Neoadjuvant and Adjuvant Therapy?
It’s simple: neoadjuvant therapy happens before surgery. Adjuvant therapy happens after. Both are systemic treatments-like chemotherapy, immunotherapy, or targeted drugs-that travel through your whole body to kill cancer cells you can’t see.
Neoadjuvant therapy is like testing the waters. You get a few rounds of treatment, then the surgeon removes the tumor. Doctors can look at the removed tissue and see exactly how well the drugs worked. Did the tumor shrink? Did it disappear completely? That answer tells them what’s likely to happen next-and whether they need to change course after surgery.
Adjuvant therapy is more like cleanup. Surgery removes the visible tumor, but there might be tiny clusters of cancer cells hiding elsewhere. Adjuvant treatment aims to wipe those out before they grow back. It’s preventative, not predictive.
Why Neoadjuvant Therapy Is Changing the Game
For years, adjuvant therapy was the standard. But around 2020, things started shifting-especially in lung and breast cancer. The reason? Neoadjuvant therapy gives you real-time feedback.
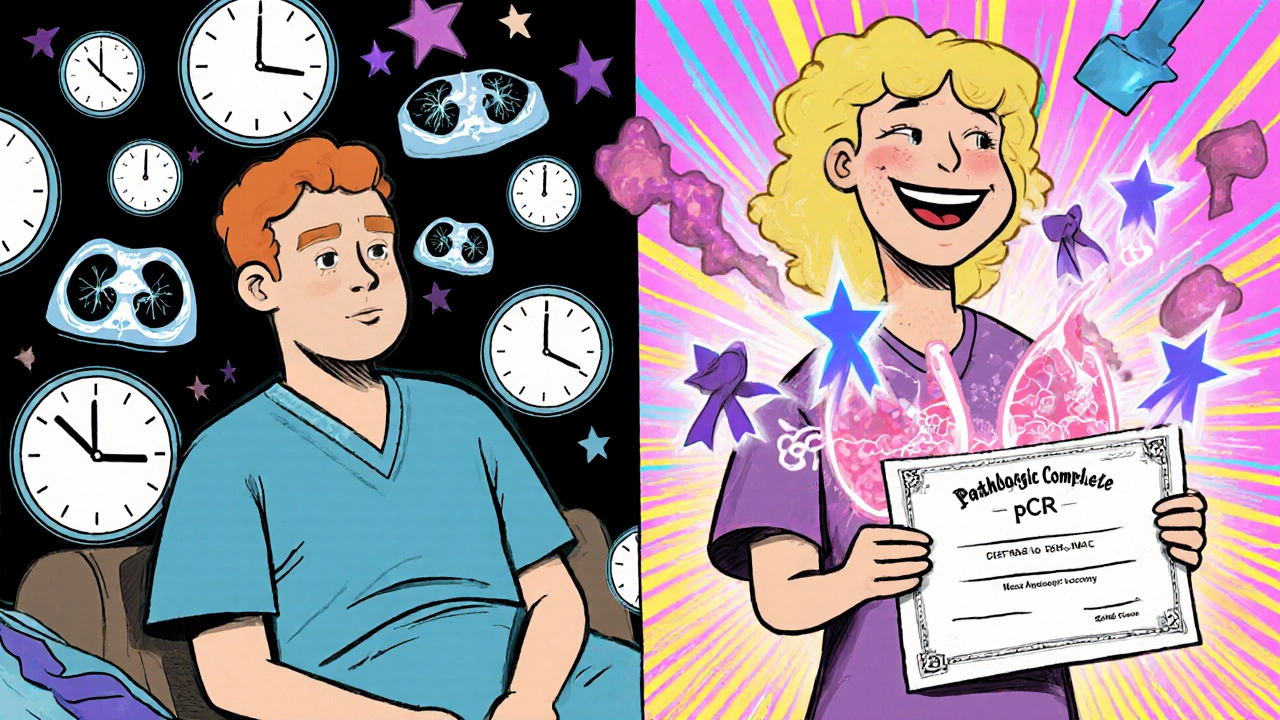
In a landmark 2022 study called CheckMate 816, patients with stage II-III non-small cell lung cancer got either chemotherapy alone or chemotherapy plus the immunotherapy drug nivolumab before surgery. The results were striking: 24% of patients who got the combo had a pathologic complete response (pCR)-meaning no living cancer cells were found in the tumor after treatment. Only 2.2% of those who got chemo alone had that result.
That’s not just a number. For patients, seeing that their tumor responded so well before surgery is a huge psychological win. One patient on a cancer forum wrote: “My oncologist said 90% of the tumor was dead. That gave me hope I didn’t have before.”
Neoadjuvant therapy also helps surgeons. If a tumor shrinks enough, they might be able to do a less invasive operation-like saving a lung lobe instead of removing the whole lung. It also tackles micrometastases early, before surgery can spread cells around.
When Adjuvant Therapy Still Makes Sense
That doesn’t mean adjuvant therapy is outdated. For many patients, especially those with smaller tumors or slower-growing cancers, it’s still the best path.
Some people don’t want to delay surgery. They’re anxious to get the tumor out as soon as possible. Others have tumors that don’t respond well to drugs upfront. In those cases, removing the tumor first and then treating the leftovers is safer and more predictable.
For breast cancer, studies show that patients with triple-negative or HER2-positive subtypes benefit most from neoadjuvant chemo. But for hormone-receptor-positive cancers, especially in older patients, doctors often still start with surgery, then add hormone therapy afterward. The difference in survival between the two approaches is small-but the side effects aren’t.
The Big Shift: Neoadjuvant-Only Is Gaining Ground
Here’s where things get really interesting. For a long time, the plan was: neoadjuvant therapy, then surgery, then more therapy (adjuvant). But new data is challenging that.
A 2024 meta-analysis of over 3,200 patients found that adding adjuvant immunotherapy after neoadjuvant treatment didn’t improve survival-but it did increase serious side effects. Patients on the full combo had nearly 30% chance of grade 3 or higher side effects like lung inflammation, liver damage, or severe fatigue. Those who stopped after neoadjuvant therapy? Around 18%.
Dr. Mark Awad from Dana-Farber put it bluntly in an ASCO talk: “The neoadjuvant-only approach may be the optimal strategy for early-stage lung cancer.”
That’s not a blanket rule. It depends on your tumor’s biology. If your tumor had a strong response to neoadjuvant treatment-like a pCR-you might not need more. But if the tumor barely changed? That’s a red flag. You might still need adjuvant therapy to reduce the risk of relapse.
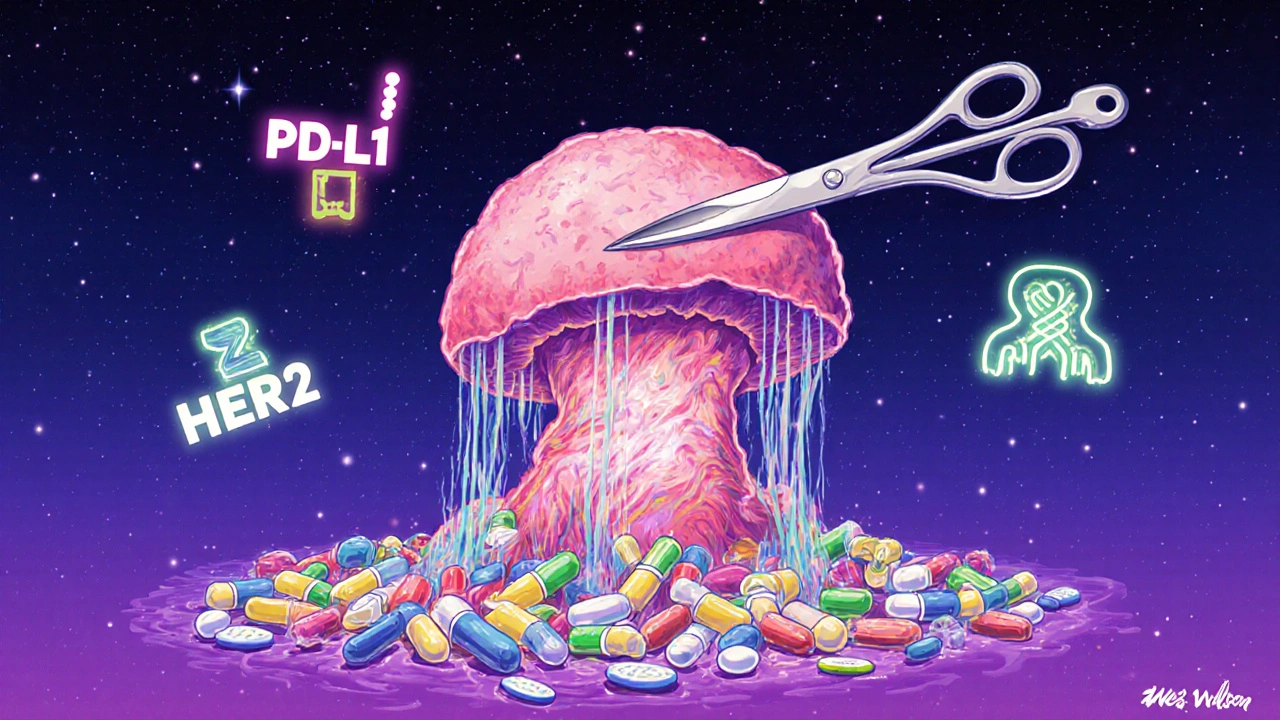
How Doctors Decide: Biomarkers and Response
This isn’t guesswork. Doctors use specific tools to guide decisions.
- PD-L1 expression: In lung cancer, if your tumor has PD-L1 levels of 1% or higher, you’re more likely to respond to immunotherapy. That makes neoadjuvant combo therapy a strong option.
- Pathologic complete response (pCR): After surgery, the pathologist checks the tumor tissue. No viable cancer cells? That’s a pCR. Patients with pCR have significantly better long-term survival-sometimes 70-80% at five years.
- Tumor subtype: Triple-negative breast cancer? Neoadjuvant chemo is standard. HER2-positive? Neoadjuvant chemo plus targeted drugs like trastuzumab. Hormone-positive? Often surgery first, then endocrine therapy.
Doctors also use imaging-CT scans, PET scans-to track tumor size before and after treatment. But scans can lie. That’s why the final pathology report after surgery is the gold standard.
What About Side Effects and Timing?
Neoadjuvant therapy usually means 3 to 4 cycles of treatment over 9 to 12 weeks before surgery. That’s a long time to wait, especially if you’re in pain or anxious. About 10-15% of patients experience delays because of side effects like low blood counts, nerve damage, or infections.
Adjuvant therapy starts after you’ve healed from surgery-usually 4 to 6 weeks later-and continues for 4 to 6 months. It’s less disruptive to your recovery, but you’re still dealing with fatigue, nausea, or immune-related issues.
One trade-off: Neoadjuvant therapy means you’re getting treatment while your body is still strong. Adjuvant therapy comes when you’re still recovering from surgery. For some, that’s harder.
Who Gets Which Treatment? Guidelines in 2025
The National Comprehensive Cancer Network (NCCN) updated its guidelines in early 2024. Here’s what they recommend:
- Non-small cell lung cancer (NSCLC): Neoadjuvant chemoimmunotherapy for stage IB (tumor ≥4 cm) to IIIA. Surgery should happen 3 to 6 weeks after the last dose.
- Triple-negative breast cancer: Neoadjuvant chemo is standard for stage II or III. If pCR is achieved, no additional chemo is needed.
- HER2-positive breast cancer: Neoadjuvant chemo plus trastuzumab and pertuzumab. Surgery after 4-6 cycles.
- Hormone-receptor-positive breast cancer: Usually surgery first, then hormone therapy for 5-10 years. Neoadjuvant is only considered if the tumor is large or growing fast.
But guidelines aren’t law. They’re starting points. Your oncologist will consider your age, overall health, tumor genetics, and personal preferences.

The Real-World Gap: Access and Expertise
Here’s the catch: Not every hospital can do this well.
Academic centers have multidisciplinary teams-surgeons, oncologists, radiologists, pathologists-all talking daily. Community hospitals? Only 58% have formal neoadjuvant pathways, according to a 2023 study. That means patients in smaller towns might not even be offered neoadjuvant options.
Also, interpreting response isn’t easy. You need pathologists trained in grading tumor response using systems like Miller-Payne for breast cancer or the American College of Pathologists system for lung. Not every lab does this consistently.
If you’re considering neoadjuvant therapy, ask: “Does your team have experience with this? Do they review cases together? Can they explain what a pCR means for me?”
What’s Next? The Future of Sequencing
Doctors aren’t stopping here. The next big thing is using circulating tumor DNA (ctDNA)-tiny pieces of cancer DNA floating in your blood-to guide treatment.
After surgery, if ctDNA is still detectable, it means cancer cells are still hiding. That’s a sign you need more treatment. If it’s gone? You might be able to avoid adjuvant therapy entirely.
Trials like NeoADAURA (for EGFR-mutant lung cancer) and KEYNOTE-867 are testing exactly this. Early results suggest ctDNA could become the new way to decide who needs more drugs-and who doesn’t.
By 2030, experts predict that 70% of early-stage lung cancer patients will get neoadjuvant therapy tailored to their tumor’s genetic profile. Survival rates could jump from 65% to over 75%.
That’s not science fiction. It’s happening now.
What Should You Do?
If you’re facing surgery for cancer, ask these questions:
- Is neoadjuvant therapy an option for my type and stage of cancer?
- What’s my chance of a pathologic complete response?
- Will we test for PD-L1 or other biomarkers?
- What happens if the tumor doesn’t shrink?
- Can we monitor ctDNA after surgery?
- Do you have a team that handles neoadjuvant cases regularly?
There’s no one-size-fits-all answer. But knowing the difference between neoadjuvant and adjuvant therapy gives you power. It lets you ask better questions, make smarter choices, and take part in your own care.
The goal isn’t just to survive. It’s to survive with fewer side effects, less fear, and more confidence that you got the right treatment at the right time.
Is neoadjuvant therapy better than adjuvant therapy?
Neither is universally better. Neoadjuvant therapy lets doctors see how your tumor responds before surgery and can shrink tumors to make surgery easier. Adjuvant therapy cleans up leftover cancer cells after surgery. Survival outcomes are similar, but neoadjuvant offers the advantage of real-time feedback. For some cancers-like triple-negative breast cancer or stage II-III lung cancer-neoadjuvant is now preferred because of improved response rates and the ability to tailor next steps.
How long does neoadjuvant therapy last before surgery?
Typically, neoadjuvant therapy lasts 3 to 4 cycles over 9 to 12 weeks. For lung cancer with immunotherapy and chemo, it’s often 3 cycles every 3 weeks. Surgery is scheduled 3 to 6 weeks after the last dose to allow your body to recover from side effects like low blood counts or fatigue. Timing is critical-too soon and you risk poor healing; too late and the cancer might start growing again.
What is a pathologic complete response (pCR), and why does it matter?
A pathologic complete response (pCR) means no living cancer cells are found in the tumor tissue after surgery. It’s the strongest indicator that the treatment worked. Patients who achieve pCR-especially in triple-negative breast cancer or lung cancer-have significantly better long-term survival. In the CheckMate 816 trial, patients with pCR had over 80% survival at three years, compared to about 50% for those without pCR. It’s not just a lab result-it’s a predictor of your future.
Do I need adjuvant therapy after neoadjuvant treatment?
Not always. New data from 2024 shows that for many patients with lung cancer, stopping after neoadjuvant therapy gives the same survival as adding more treatment afterward-but with fewer side effects. If your tumor had a strong response (like pCR), you may not need adjuvant therapy. But if your tumor barely changed, your doctor may still recommend it. The decision is based on your pathology report, biomarkers, and overall risk.
Can I choose adjuvant therapy even if neoadjuvant is available?
Yes, and it’s a valid choice. Some patients prefer to get surgery right away and avoid the uncertainty of waiting. Others have tumors that don’t respond well to drugs upfront. If you’re anxious about delaying surgery, or if your cancer is slow-growing, adjuvant therapy is still a proven option. The goal is to match the treatment to your personal situation-not just follow the latest trend.
Are there risks to neoadjuvant therapy?
Yes. The main risk is that your cancer could grow or spread during the 2-3 months of treatment before surgery. About 5-10% of lung cancer patients experience disease progression on neoadjuvant therapy. Side effects like fatigue, low blood counts, or immune-related reactions (like lung inflammation) can delay surgery. About 10-15% of patients need to postpone their operation because of these issues. That’s why close monitoring with scans and blood tests is essential.
What if my tumor doesn’t respond to neoadjuvant therapy?
That’s a critical moment. If your tumor shows little or no shrinkage, your oncology team will use that information to adjust your plan. You may still have surgery, but your doctors will likely recommend stronger or different adjuvant therapy afterward. In some cases, they might suggest clinical trials with newer drugs. The fact that the tumor didn’t respond tells you something important: it’s resistant to that treatment. That’s valuable data-and it helps guide your next steps.
Is neoadjuvant therapy only for lung and breast cancer?
No, but those are the most common. It’s also used in rectal cancer, esophageal cancer, and some sarcomas. For example, in locally advanced rectal cancer, neoadjuvant chemoradiation is standard before surgery. Research is expanding into other cancers like melanoma and ovarian cancer. The key is whether the tumor is resectable and whether systemic therapy can meaningfully shrink it. Your oncologist will know if it’s appropriate for your specific diagnosis.

So let me get this straight-we’re giving people chemo BEFORE cutting them open now? Like, just to see if the tumor is scared? 😂 I’m just here for the drama, but also… this is wild.
I’ve seen this play out with my sister-stage III triple-negative breast cancer. She did neoadjuvant chemo, got a pCR, and skipped the rest. The relief on her face after surgery? Priceless. No more waiting for the other shoe to drop. It’s not just science-it’s peace of mind.
Still, not everyone gets access to this. My local hospital didn’t even mention it until I pushed. If you’re in a community setting, ask. Ask again. And bring a notebook.
Neoadjuvant is the future but it’s not magic. I’ve had patients where the tumor barely budged and they still went into surgery thinking they ‘beat it’-only to find out the pathology report says otherwise. That’s why pCR matters. Not just because it sounds cool, but because it actually predicts survival
Also ctDNA is going to change everything. By 2030 we’ll be doing blood tests instead of biopsies. Imagine that. No more invasive scans just to check if the drugs worked.
And yes, side effects are real. One guy I knew had to delay surgery for 6 weeks because of neuropathy. He cried in the waiting room. This isn’t just data-it’s human.
Just saw this and had to say-this is one of the clearest explainers I’ve read on this topic. 🙌
My uncle had NSCLC and got the nivolumab combo. Got pCR. No adjuvant. Now he’s hiking in Colorado. Three years out. No chemo, no scans, no fear. Just life.
Doctors need to stop treating this like a one-size-fits-all checklist. Biomarkers, patient goals, access-all matter. And yeah, if your hospital doesn’t have a tumor board reviewing cases? Run. Not metaphorically. Literally. Find a center that does.
Also-thank you for mentioning ctDNA. That’s the real game changer coming. We’re not just treating tumors anymore. We’re tracking ghosts.
Why are we wasting time with all this fancy science when in India we just give chemo and cut? Why do Americans need 10 tests before they even touch a knife? We fix problems not talk about them. This is overcomplicated. You got cancer? Cut it out. Then kill what’s left. Done. No need for all this neaodjuvant bullsh*t. Also why do you need a whole team? One good doctor is enough. Stop being so soft.
Yash, I hear you. Simplicity has its place. But cancer isn’t a broken pipe. It’s a living, evolving system. What works for one person can kill another. The fact that we’re learning to personalize this? That’s progress. Not weakness.
I’ve had friends who got surgery first and then had to go back for more chemo because the cancer came back in places no scan could see. That’s the nightmare we’re trying to avoid.
It’s not about being soft. It’s about being smarter. And yeah, access is a huge problem. But that’s a system issue-not a reason to ignore the science.
Thank you for writing this. I’m a nurse in oncology and I see how scared people are when they’re told ‘we’ll treat you before surgery.’ They think it means they’re worse off. But it’s the opposite-it means we’re trying to give them the best shot.
One patient last month asked me, ‘Will I still be me after this?’ I didn’t have a good answer. But now I do. You’ll be you. Just with a little less fear. And that’s everything.
Also-please, if you’re reading this and your oncologist hasn’t mentioned pCR or ctDNA? Ask. Don’t wait for them to bring it up. You deserve to know all your options.
While the clinical data supporting neoadjuvant therapy is compelling, particularly in non-small cell lung cancer and triple-negative breast cancer, it is imperative to recognize that the decision-making framework remains highly individualized. The presence of a pathologic complete response, as validated by rigorous histopathological analysis, serves as a robust prognostic indicator; however, the absence thereof does not inherently preclude favorable outcomes, particularly when adjuvant modalities are appropriately tailored to residual disease burden. Furthermore, the logistical and infrastructural demands of implementing neoadjuvant protocols-including multidisciplinary coordination, standardized response assessment, and access to molecular diagnostics-remain substantial barriers in non-academic settings. As circulating tumor DNA assays transition from investigational tools to clinically validated biomarkers, the paradigm may shift toward dynamic, real-time decision-making, thereby minimizing overtreatment while preserving therapeutic efficacy. The ethical imperative lies not in the adoption of novel strategies per se, but in ensuring equitable access to evidence-based care regardless of geographic or socioeconomic determinants.
Ugh. I knew this post would be full of ‘experts’ who think they’re saving lives by adding more steps. Newsflash: most people just want to get the tumor out and move on. All this ‘biomarkers’ and ‘ctDNA’ nonsense? It’s just profit-driven overtesting. You think a 70-year-old woman with HR+ breast cancer needs six months of chemo before surgery? No. She needs peace. And a quiet room. Not a clinical trial.
And don’t even get me started on the ‘team’ thing. One doctor. One opinion. That’s all you need. The rest is just consultants billing your insurance.
Also-why are we pretending this is new? My dad had this in 2008. Same thing. They called it ‘pre-op chemo.’ Now it’s ‘neoadjuvant.’ Fancy word. Same treatment. Same results. Stop marketing fear.