Generic Drugs: What They Are, How They Save Money, and What You Need to Know
When you hear generic drugs, pharmaceutical products that contain the same active ingredients as brand-name medicines but are sold under their chemical name. Also known as pharmaceutical generics, they are the backbone of affordable healthcare in countries with public drug programs like Australia’s PBS and in the U.S. after Hatch-Waxman Act approvals. They’re not cheaper because they’re weaker—they’re cheaper because they don’t carry the marketing, R&D, or patent costs of the original brand. The FDA and global regulators require them to match the brand-name drug in dosage, strength, route of administration, and performance. If your doctor prescribes a generic, you’re getting the exact same medicine—just without the fancy packaging or TV ads.
Many people worry that generics are risky or less effective, but that’s not true. What actually matters is the active ingredient, the chemical compound responsible for the drug’s effect. Whether it’s atenolol for high blood pressure or fluoxetine for depression, the molecule in the generic version is identical to the one in the brand-name version. The differences are in fillers, dyes, or coatings—things that don’t affect how the drug works. What does matter is how the drug is made. The same strict standards apply to generic manufacturers as to big pharma. In fact, many brand-name companies make their own generics under different labels. And when it comes to complex drugs like those used in cancer treatment or autoimmune conditions, the ANDA litigation, the legal process that allows generic manufacturers to challenge patents before bringing their version to market ensures only truly equivalent drugs get approved.
It’s not just about saving money—it’s about access. In Australia, the Pharmaceutical Benefits Scheme, a government program that subsidizes prescription medications to make them affordable relies on generics to cover millions of prescriptions. In the U.S., the Federal Circuit Court, the court that handles all pharmaceutical patent disputes decides when generics can enter the market. These systems work because generics are trusted. You’ll find them in every pharmacy, in every insurance formulary, and in every public health plan. And if you’ve ever wondered why some drugs suddenly drop in price after a few years, that’s the patent expiring—and generics stepping in.
But not all generics are created equal in perception. Some people still hesitate because of myths—like generics take longer to work or cause more side effects. They don’t. Others worry about switching from a brand they’ve used for years. That’s valid, and your doctor should help you decide. But if cost is a barrier, switching to a generic isn’t a compromise—it’s a smart choice backed by science, regulation, and real-world use by millions.
Below, you’ll find real stories and facts about how generic drugs shape access, pricing, and safety—from how Australia’s PBS keeps meds affordable, to how patent battles delay or speed up generic entry, to what you should know before swapping your brand-name pill for a cheaper version. No fluff. Just what works, what doesn’t, and what you need to know to get the most out of your prescription.
Authorized generics are identical to brand-name drugs in every way except the label. Learn how they work, why they're safer than regular generics for some patients, and how to get them at a lower cost.
The FDA's ANDA process lets generic drugs reach the market faster and cheaper by proving they work the same as brand-name drugs. Learn how it works, why it saves billions, and what it means for your prescriptions.
Pharmacokinetic studies are the primary method used to prove generic drugs are equivalent to brand-name versions, measuring how the body absorbs and processes the drug. But they're not foolproof-especially for complex or narrow therapeutic index drugs.
Generic drugs save Americans over $330 billion a year, but brand manufacturers face massive revenue losses when patents expire. Learn how pay-for-delay deals, PBMs, and patent tricks are reshaping drug pricing-and who really pays the cost.
Pharmacists use Medication Therapy Management to optimize generic drug use, improve adherence, and cut costs. Learn how MTM works, why generics matter, and how to access this free service.
Generic drugs are just as effective as brand-name versions, saving patients up to 85% on costs. Learn how bioequivalence works, when to be cautious, and why generics are the smart choice for most people.
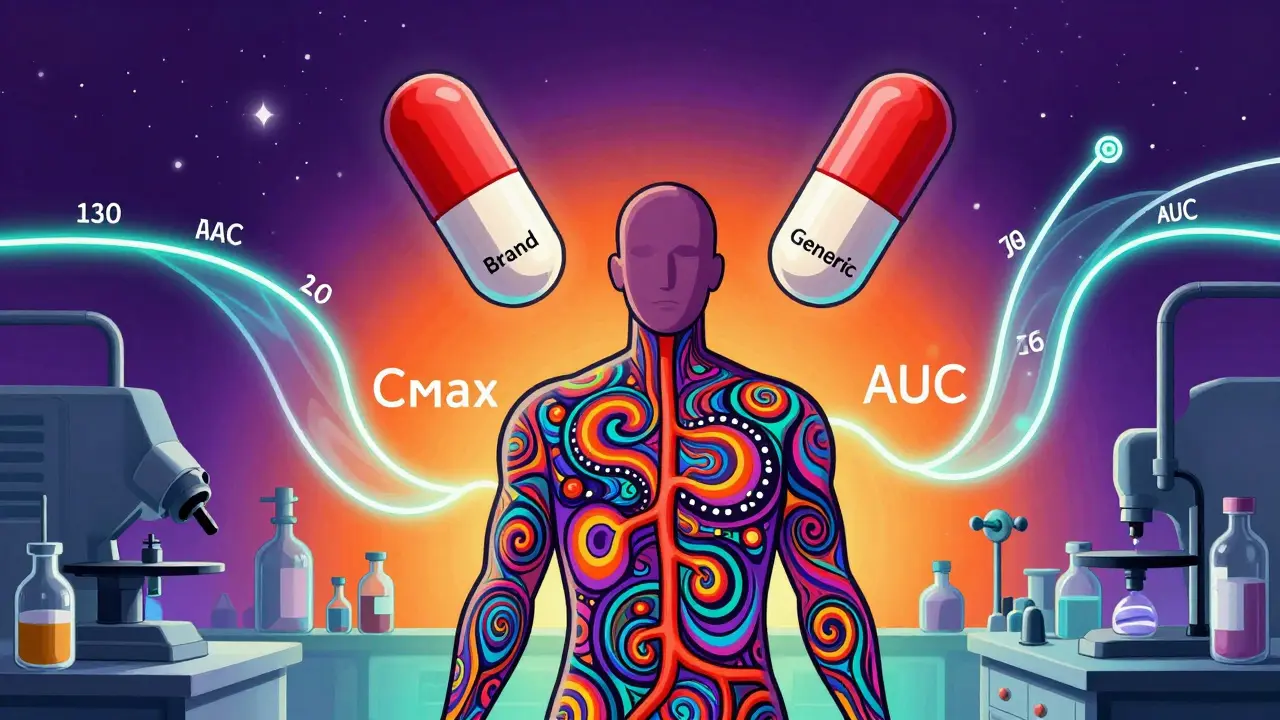
Bioavailability studies ensure generic drugs work like brand-name versions by measuring how much of the drug enters your bloodstream. Here’s how the FDA uses AUC and Cmax to approve generics safely and affordably.