Category: Medications - Page 2
Authorized generics are identical to brand-name drugs in every way except the label. Learn how they work, why they're safer than regular generics for some patients, and how to get them at a lower cost.
Proton pump inhibitors can block absorption of some antifungals like itraconazole, making treatments fail. Fluconazole is safe to use with PPIs, but voriconazole needs monitoring. New research even suggests PPIs may boost antifungal power.
The FDA's ANDA process lets generic drugs reach the market faster and cheaper by proving they work the same as brand-name drugs. Learn how it works, why it saves billions, and what it means for your prescriptions.
As people age, liver and kidney changes slow drug processing, increasing the risk of side effects and hospitalizations. Learn how these changes affect common medications and what you can do to stay safe.
Learn how antiemetics, antihistamines, and steroids prevent dangerous reactions during CT scans and chemotherapy. Discover who needs pre-medication, the best drug combinations, and how hospitals are reducing errors.
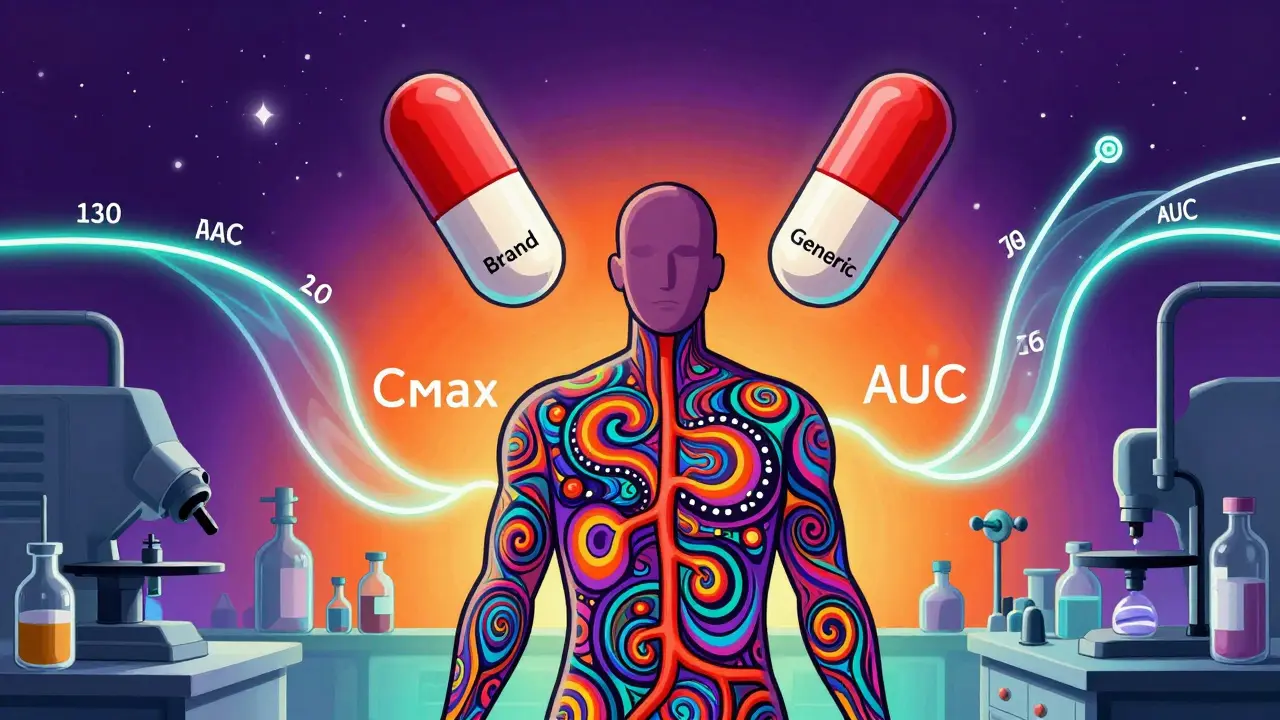
Pharmacokinetic studies are the primary method used to prove generic drugs are equivalent to brand-name versions, measuring how the body absorbs and processes the drug. But they're not foolproof-especially for complex or narrow therapeutic index drugs.
When no generic exists, brand-name drugs can cost tens of thousands a month. Patient assistance programs can cover the full cost-but only if you know how to apply and avoid hidden traps like accumulator adjustments. Learn how to get free or low-cost meds when there's no cheaper option.
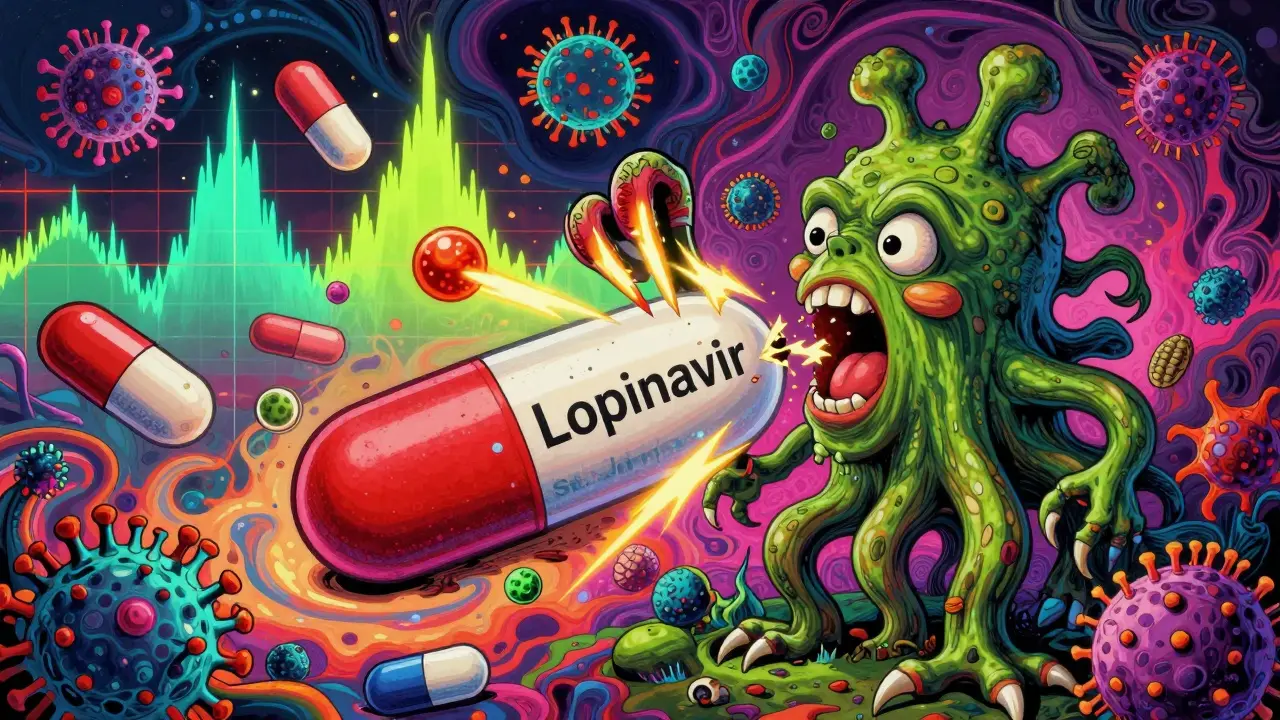
St. John’s Wort can slash HIV protease inhibitor levels by up to 81%, leading to treatment failure and drug resistance. This interaction is well-documented, dangerous, and still happening-despite decades of warnings.
Generic drugs save Americans over $330 billion a year, but brand manufacturers face massive revenue losses when patents expire. Learn how pay-for-delay deals, PBMs, and patent tricks are reshaping drug pricing-and who really pays the cost.
Pharmacists use Medication Therapy Management to optimize generic drug use, improve adherence, and cut costs. Learn how MTM works, why generics matter, and how to access this free service.